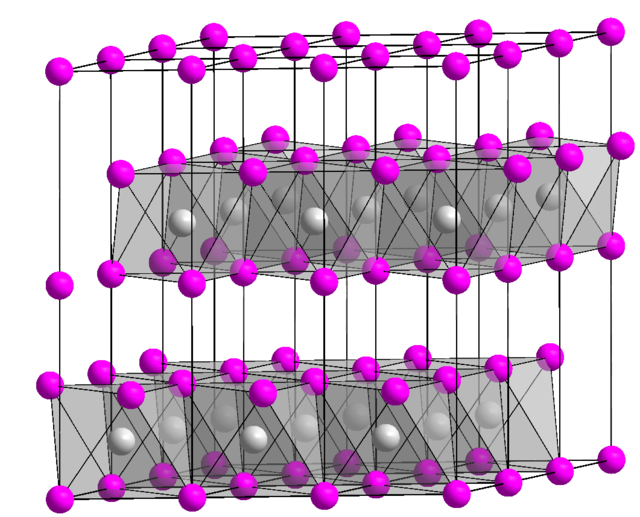
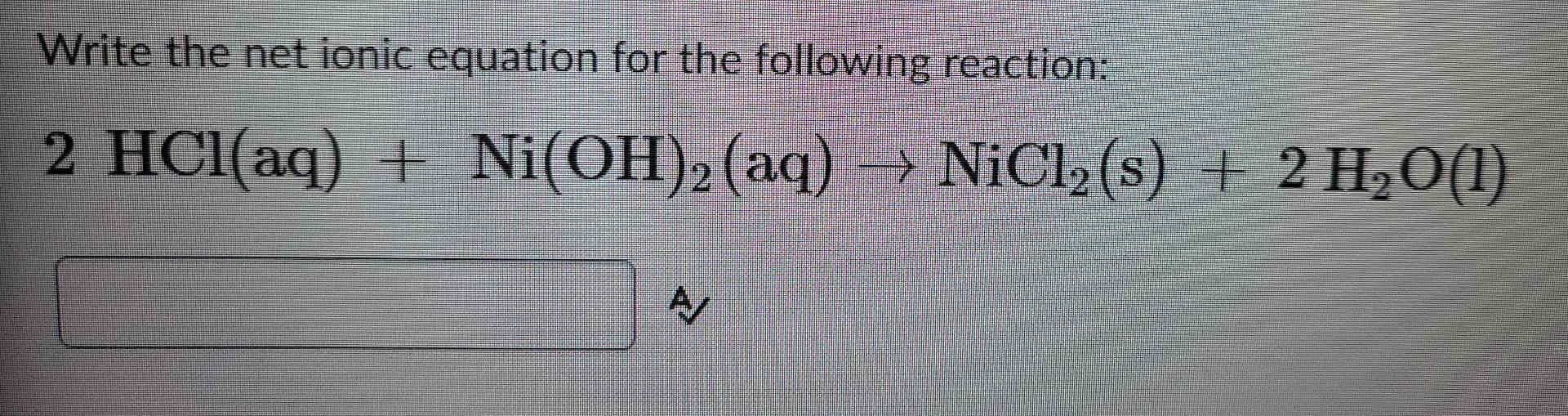5 Six methods of preparing Ni(OH) 2. (a) Basification of a nickel(II)... | Download Scientific Diagram

Electrochemical partial reduction of Ni(OH)2 to Ni(OH)2/Ni via coupled oxidation of an interfacing NiAl intermetallic compound for robust hydrogen evolution - ScienceDirect

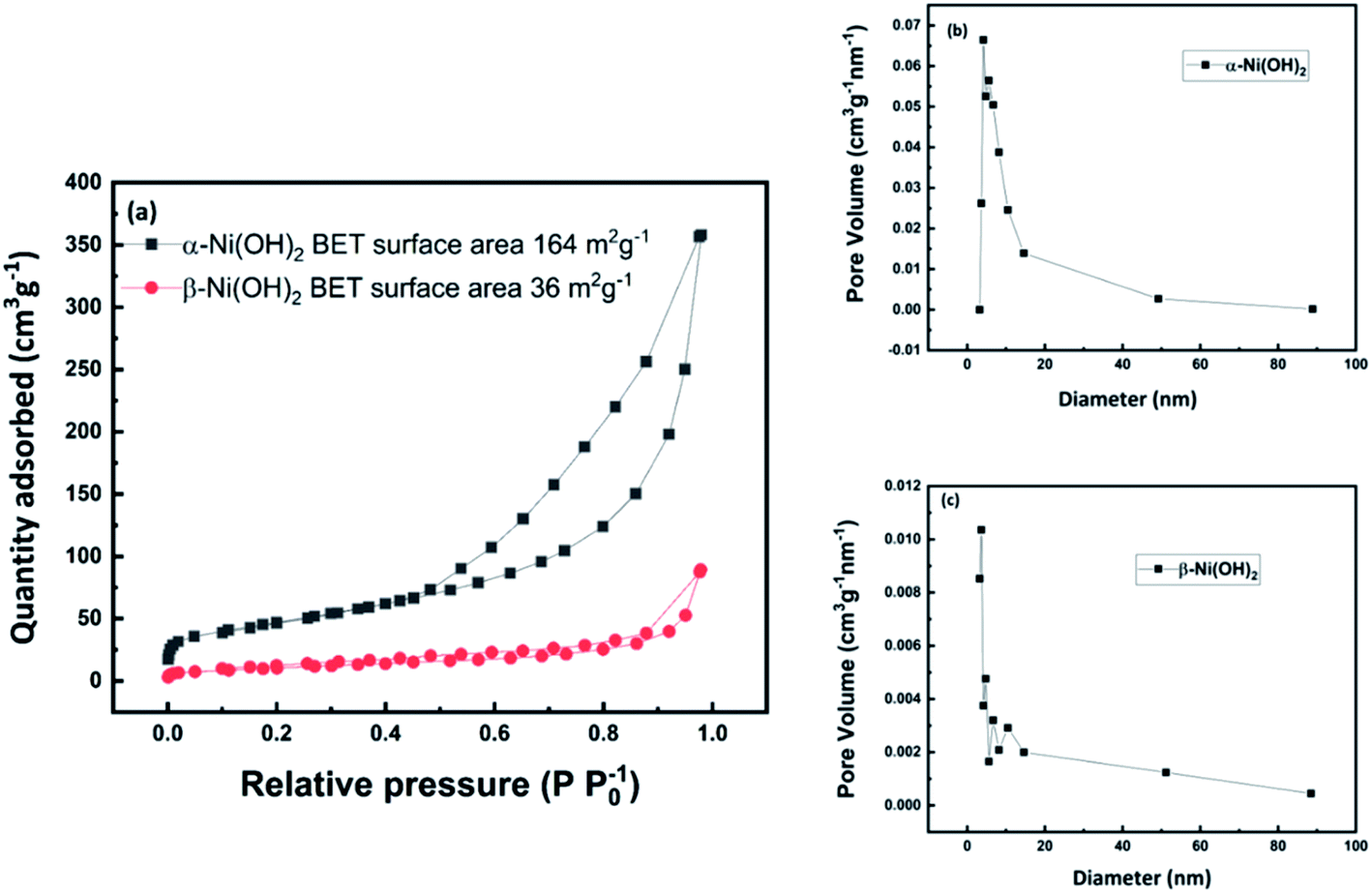
Triple Functions of Ni(OH)2 on the Surface of WN Nanowires Remarkably Promoting Electrocatalytic Activity in Full Water Splitting | ACS Catalysis
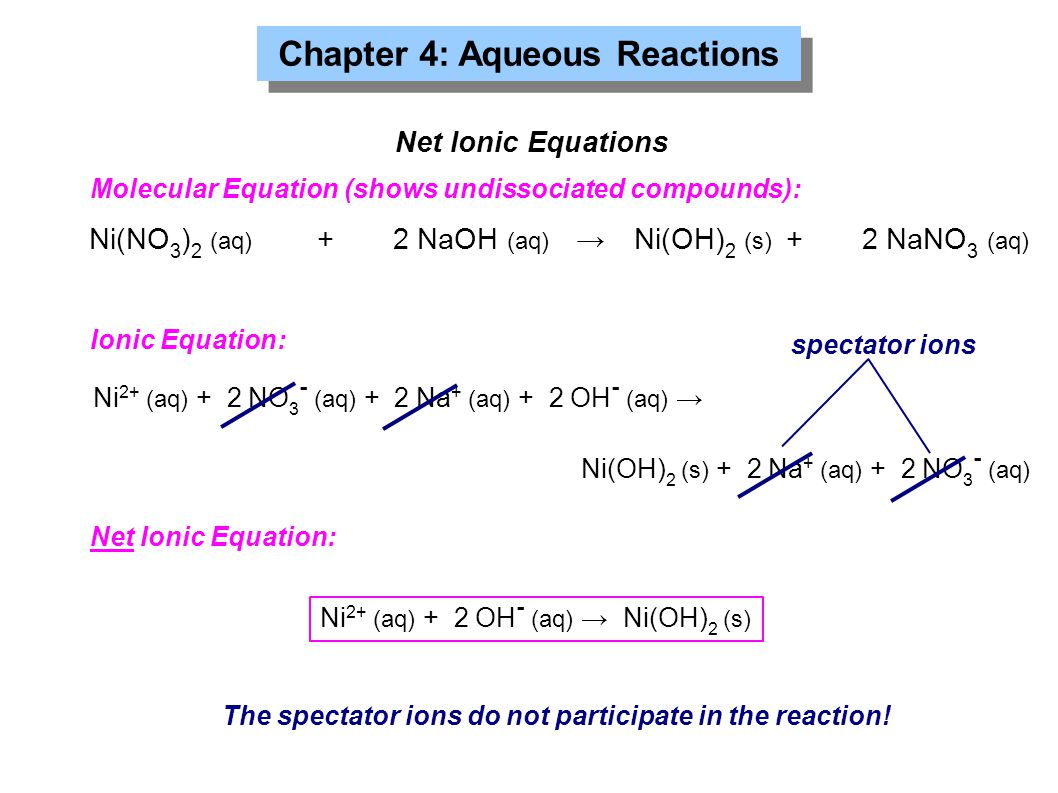
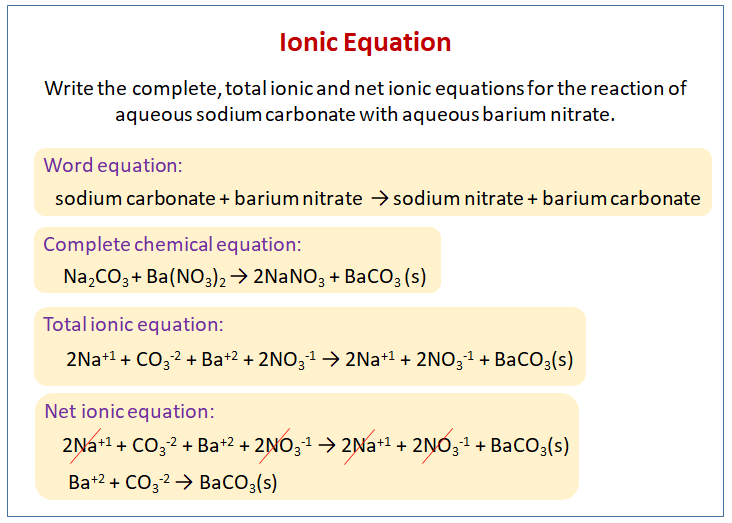
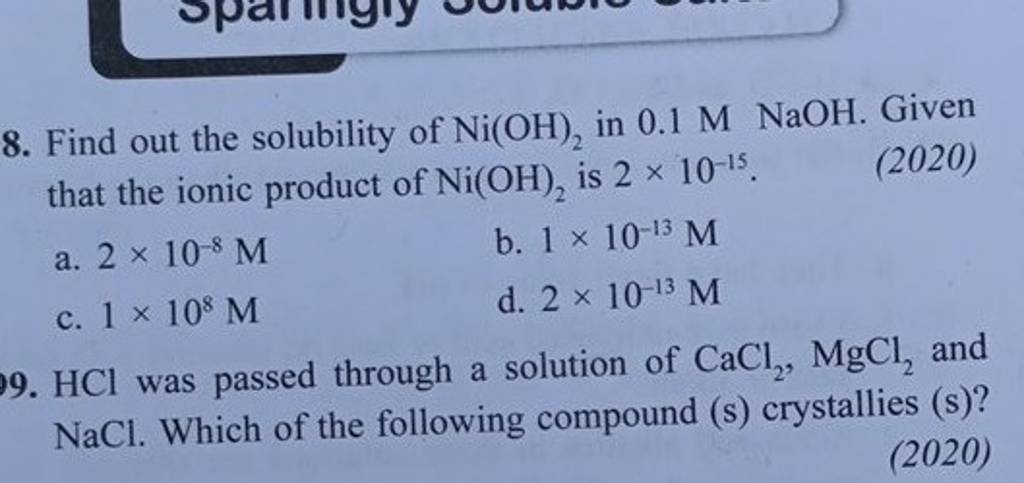
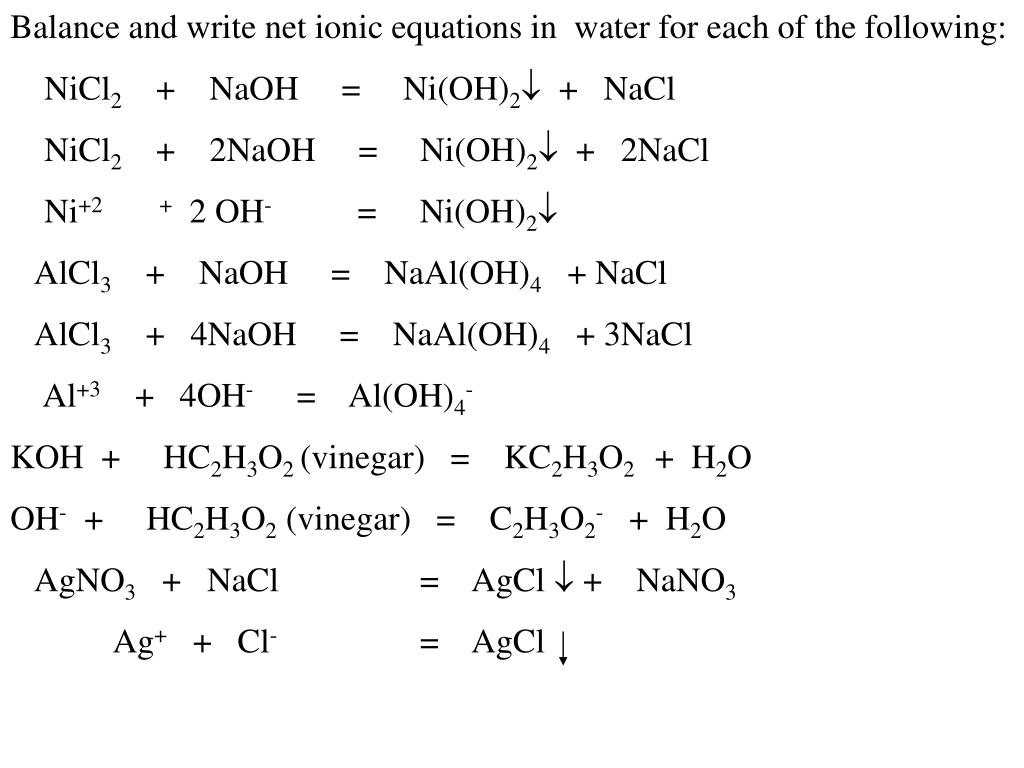
Chapter 4: Aqueous Reactions Solution: Solvent: substance present in the larger amount Solute: substance(s) dissolved in solvent, generally present in. - ppt download

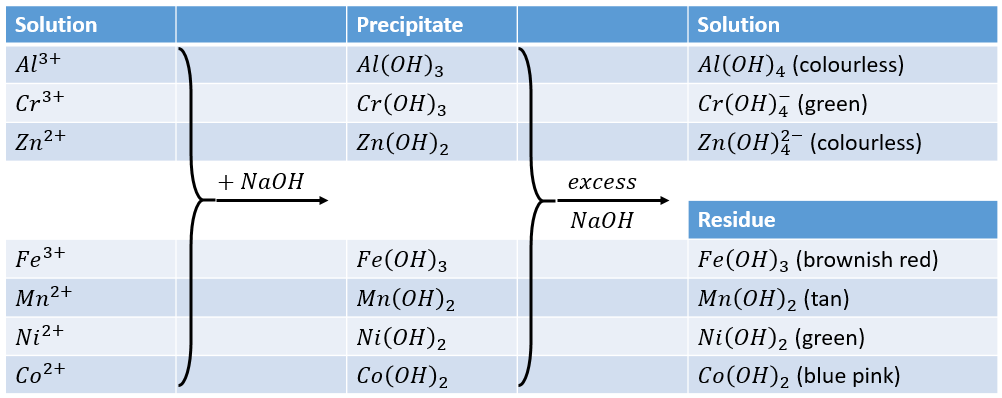
Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes

Triple Functions of Ni(OH)2 on the Surface of WN Nanowires Remarkably Promoting Electrocatalytic Activity in Full Water Splitting | ACS Catalysis